Page 50 - CUA 2020_Onco_Prostate
P. 50
2020 CUA Abstracts
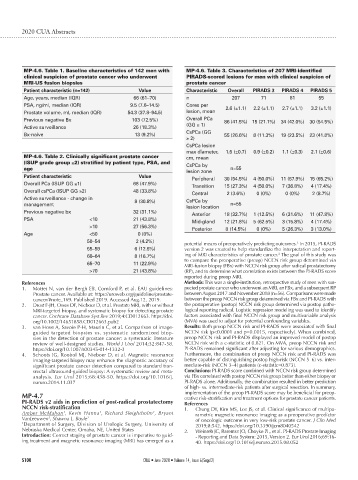
MP-4.6. Table 1. Baseline characteristics of 142 men with MP-4.6. Table 3. Characteristics of 207 MRI-identified
clinical suspicion of prostate cancer who underwent PIRADS-scored lesions for men with clinical suspicion of
MRI-US fusion biopsies prostate cancer
Patient characteristic (n=142) Value Characteristic Overall PIRADS 3 PIRADS 4 PIRADS 5
Age, years, median (IQR) 66 (61–70) n 207 71 81 55
PSA, ng/ml, median (IQR) 9.5 (7.6–14.5) Cores per 2.6 (±1.1) 2.2 (±1.1) 2.7 (±1.1) 3.2 (±1.1)
Prostate volume, ml, median (IQR) 54.3 (37.8–94.5) lesion, mean
Previous negative Bx 103 (72.5%) Overall PCa 86 (41.5%) 15 (21.1%) 34 (42.0%) 30 (54.5%)
(GG ≥ 1)
Active surveillance 26 (18.3%)
CsPCa (GG
Bx-naive 13 (9.2%) 55 (26.6%) 8 (11.3%) 19 (23.5%) 23 (41.8%)
≥ 2)
CsPCa lesion
max diameter, 1.5 (±0.7) 0.9 (±0.2) 1.1 (±0.3) 2.1 (±0.6)
MP-4.6. Table 2. Clinically significant prostate cancer cm, mean
(ISUP grade group ≥2) stratified by patient type, PSA, and CsPCa by
age n=55
lesion zone
Patient characteristic Value
Peripheral 30 (54.5%) 4 (50.0%) 11 (57.9%) 15 (65.2%)
Overall PCa (ISUP GG ≥1) 68 (47.9%)
Transition 15 (27.3%) 4 (50.0%) 7 (36.8%) 4 (17.4%)
Overall csPCa (ISUP GG ≥2) 48 (33.8%)
Central 2 (3.6%) 0 (0%) 0 (0%) 2 (8.7%)
Active surveillance - change in 8 (30.8%) CsPCa by
management n=55
lesion location
Previous negative bx 32 (31.1%)
Anterior 18 (32.7%) 1 (12.5%) 6 (31.6%) 11 (47.8%)
PSA <10 21 (43.8%)
Mid-gland 12 (21.8%) 5 (62.5%) 3 (15.8%) 4 (17.4%)
>10 27 (56.3%)
Posterior 8 (14.5%) 0 (0%) 5 (26.3%) 3 (13.0%)
Age <50 0 (0%)
50–54 2 (4.2%) 1
potential means of preoperatively predicting outcomes. In 2015, PI-RADS
55–59 6 (12.5%) version 2 was created to help standardize the interpretation and report-
2
60–64 8 (16.7%) ing of MRI characteristics of prostate cancer. The goal of this study was
to compare the preoperative (preop) NCCN risk group determined via
65–70 11 (22.9%)
MRI-fusion biopsy (FBx) with NCCN risk group after radical prostatectomy
>70 21 (43.8%) (RP), and to determine what correlation exists between the PI-RADS score
reported during preop MRI.
References Methods: This was a single-institution, retrospective study of men with sus-
1. Mottet N, van der Bergh EB, Cornford P, et al. EAU guidelines: pected prostate cancer who underwent an MRI, an FBx, and a subsequent RP
Prostate cancer. Available at: https://uroweb.org/guideline/prostate- between August 2017 and November 2018 (n=56). Comparisons were made
cancer/#note_169. Published 2019. Accessed Aug.12, 2019. between the preop NCCN risk group determined via FBx and PI-RADS with
2. Drost F-JH, Osses DF, Nieboer D, et al. Prostate MRI, with or without the postoperative (postop) NCCN risk group determined via postop patho-
MRI‐targeted biopsy, and systematic biopsy for detecting prostate logical reporting radical. Logistic regression modeling was used to identify
cancer. Cochrane Database Syst Rev 2019;4:CD012663. https://doi. factors associated with final NCCN risk group and multivariable analysis
org/10.1002/14651858.CD012663.pub2 (MVA) was used to adjust for potential confounding variables.
3. van Hove A, Savoie P-H, Maurin C, et al. Comparison of image- Results: Both preop NCCN risk and PI-RADS were associated with final
guided targeted biopsies vs. systematic randomized biop- NCCN risk (p<0.0001 and p=0.0015, respectively). When combined,
sies in the detection of prostate cancer: a systematic literature preop NCCN risk and PI-RADS displayed an improved model of postop
review of well-designed studies. World J Urol 2014;32:847-58. NCCN risk with a c-statistic of 0.821. On MVA, preop NCCN risk and
https://doi.org/10.1007/s00345-014-1332-3 PI-RADS remained significant after adjusting for various demographics.
4. Schoots IG, Roobol MJ, Nieboer D, et al. Magnetic resonance Furthermore, the combination of preop NCCN risk and PI-RADS was
imaging-targeted biopsy may enhance the diagnostic accuracy of better capable of distinguishing postop high-risk (NCCN 5–6) vs. inter-
significant prostate cancer detection compared to standard tran- mediate-risk (NCCN 3–4) patients (c-statistic=0.873).
srectal ultrasound-guided biopsy: A systematic review and meta- Conclusions: PI-RADS score combined with NCCN risk group determined
analysis. Eur Urol 2015;68:438-50. https://doi.org/10.1016/j. via FBx correlated with postop NCCN risk group better than either biopsy or
eururo.2014.11.037 PI-RADS alone. Additionally, the combination resulted in better prediction
of high- vs. intermediate-risk patients after surgical resection. In summary,
MP-4.7 implementation of the preop PI-RADS score may be beneficial for preop-
erative risk-stratification and treatment options for prostate cancer patients.
PI-RADS v2 aids in prediction of post-radical prostatectomy References
NCCN risk-stratification 1. Chung DY, Kim MS, Lee JS, et al. Clinical significance of multipa-
Amber McMahon , Kevin Hanna , Richard Sleightholm , Bryant rametric magnetic resonance imaging as a preoperative predictor
1
1
1
1
VanLeeuwen , Shawna L. Boyle 1 of oncologic outcome in very low-risk prostate cancer. J Clin Med
1 Department of Surgery, Division of Urologic Surgery, University of 2019;8:542. https://doi.org/10.3390/jcm8040542
Nebraska Medical Center, Omaha, NE, United States 2. Weinreb JC, Barentsz JO, Choyke PL, et al. PI-RADS Prostate Imaging
Introduction: Correct staging of prostate cancer is imperative to guid- - Reporting and Data System: 2015, Version 2. Eur Urol 2016;69:16-
ing treatment and magnetic resonance imaging (MRI) has emerged as a 40. https://doi.org/10.1016/j.eururo.2015.08.052
S100 CUAJ • June 2020 • Volume 14, Issue 6(Suppl2)